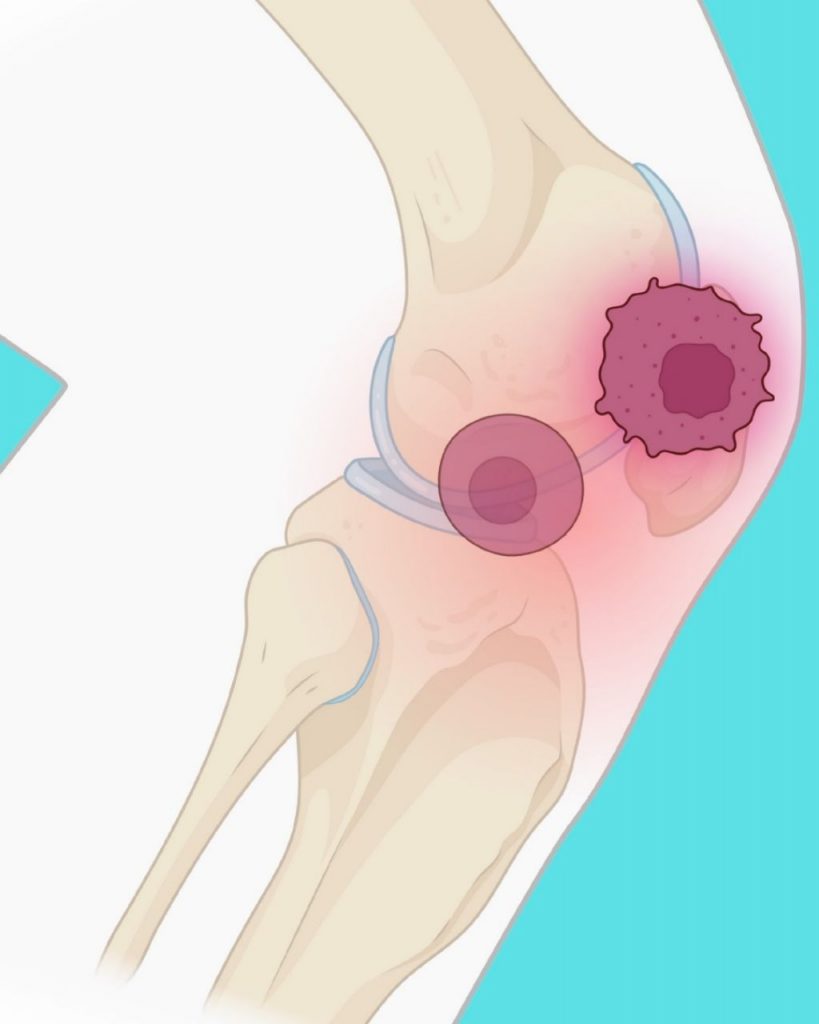
A cutting-edge MRI technique developed by researchers at Northwestern University and Stanford University could revolutionize how doctors detect and treat arthritis and other aging-related conditions. The method makes it possible, for the first time, to non-invasively visualize senescent cells—damaged cells that stop dividing and contribute to chronic inflammation and tissue degeneration.
The imaging breakthrough builds on years of foundational work by Thomas Meade, PhD, Professor of chemistry at Northwestern and Chemistry of Life Processes Institute member, whose lab engineered a specialized MRI contrast agent that activates in the presence of a biomarker enzyme commonly found in senescent cells. This “smart” agent, which remains inactive until it encounters the enzyme beta-galactosidase, allows clinicians to see where senescent cells are accumulating in the body—cells that are thought to drive diseases like osteoarthritis, Alzheimer’s, and certain cancers.
“Senescent cells are sometimes called ‘zombie cells’—they’re not dead, but they’ve stopped functioning normally and can damage surrounding tissue,” said Meade. “Until now, there hasn’t been a reliable way to locate them in living tissue. Our contrast agent changes that.”

Published May 3 in npj Imaging, the study describes how the agent was tested in animal models and tissue samples, specifically targeting senescent cells implanted in pig joints. The results show that the imaging agent illuminates only the areas where these aging cells are present, offering a highly specific and safe method for detection. Further testing is ongoing using human joint tissue removed during knee replacement surgeries.
The innovation has significant implications for patients suffering from osteoarthritis. While emerging senolytic therapies aim to eliminate senescent cells and halt disease progression, clinical trials have faced a major limitation: the inability to directly monitor whether these treatments are working inside the body. Meade’s agent may provide a solution.
“By offering a window into cellular aging, this technique could not only guide patient selection for therapies but also provide fast feedback on whether a treatment is hitting its target,” Meade said.
The concept emerged through a collaboration between Northwestern and Stanford researchers, who recognized the potential to apply Meade’s chemistry-driven contrast agent to the study of arthritis and joint degeneration. The underlying technology originated from Meade’s research into imaging agents for gene therapy monitoring.
“This is a prime example of how basic chemical research at Northwestern can be translated into medical tools with broad clinical applications,” Meade said.
Beyond arthritis, the technology may enable new insights into other age-associated diseases. Because senescent cells accumulate throughout the body over time, researchers are exploring how this imaging platform might be used to track aging, evaluate anti-aging therapies, and diagnose conditions earlier.
Funding for the project came from the National Institutes of Health and NASA, with support for both the development of the imaging agent and collaborative testing. Meade has also launched a company, PreDx, to advance this and related imaging technologies toward clinical use.
As Northwestern continues to push the boundaries of biomedical research, Meade’s work exemplifies the power of interdisciplinary science—where chemistry meets medicine—to tackle some of the most urgent challenges of aging and disease.
Main image caption: A novel MR-based molecular imaging probe developed by Northwestern and Stanford Medicine researchers lights up zombie cells in the knee. Created with BioRender.com by Vidyani Suryadevara

